International Standardization

Good manufacturing practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product. GMP covers all aspects of production; from the starting materials, premises and equipment to the training and personal hygiene of staff. WHO has established detailed guidelines for good manufacturing practice.
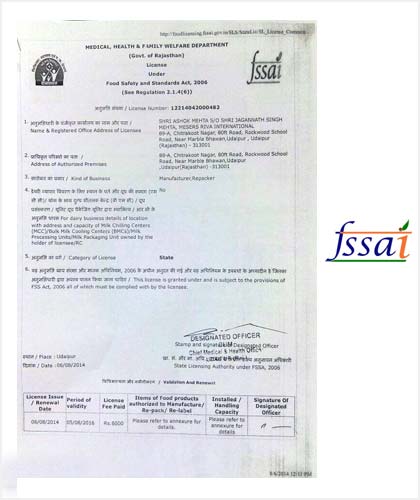
FSSAI has been established under Food Safety and Standards Act, 2006 which consolidates various acts & orders that have hitherto handled food related issues. FSSAI has been created for laying down science based standards for articles of food and to regulate their manufacture, storage, distribution, sale and import to ensure availability of safe and wholesome food for human consumption.

HACCP (Hazard Analysis and Critical Control Point) is a system that helps food business operators look at how they handle food and introduces procedures to make sure the food produced is safe to eat. It is a management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product.

ISO, founded in 1947, is a worldwide federation of national standards bodies from some countries, with one standards body representing each member country. ISO 22000:2005 specifies requirements for a food safety management system where an organization in the food chain needs to demonstrate its ability to control food safety hazards in order to ensure that food is safe at the time of human consumption.



 support@herbaldaily.in
support@herbaldaily.in











